Cracking the Code to 2025 CMS Star Ratings
Key Measures and Strategic Insights
-
September 04, 2024
-
Are you prepared for the coming additions to Centers for Medicare and Medicaid Services (“CMS”) Medicare Advantage (“MA”) Star Ratings Measures? Beginning on January 1, 2025, CMS is implementing additions and changes that will impact 2027 Star Ratings. As such, MA Plans and providers must ensure they are fully prepared to identify beneficiaries for which these new measures apply and develop strategies to address their care needs — and time is ticking. Health plans that struggle with low margins need to perform well on Star Ratings, and the potential financial repercussions of non-compliance can have a serious and lasting impact on a health plan’s profitability.1
At stake is approximately $16 billion in additional Star bonuses.2 To maintain success in the program, MA plans must continuously enhance operational capabilities, including the analytics to identify which members have Star Rating measures requiring assessment, the strategies and intervention abilities to close measures and the data operations required to report closed measure data to the CMS and the National Committee of Quality Assurance (“NCQA”).
Source3
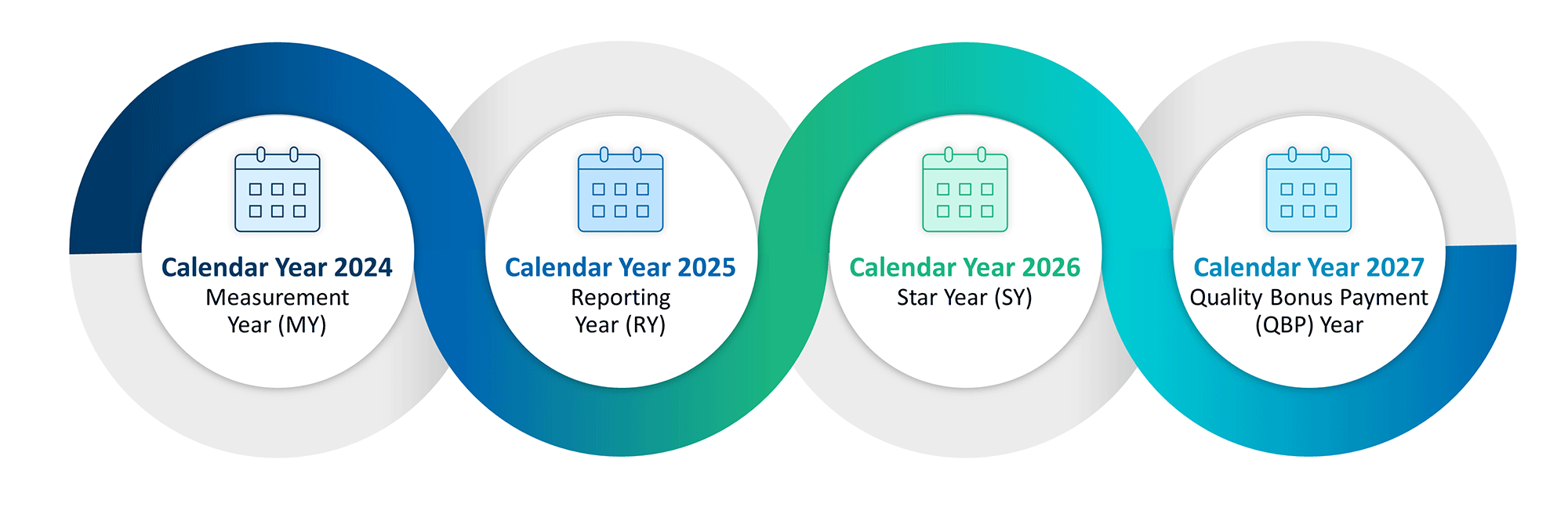
The CMS Star Ratings Cycle
Star ratings are determined through a four-year process. The first year is the measurement year (“MY”), in which plans collect performance data for various measures, though specific timing can vary — for instance, Consumer Assessment of Healthcare Providers and Systems (“CAHPS”) surveys are conducted at the start of the second year. During the second year — the reporting year (“RY”) — the CMS evaluates the previous year's performance data. The third year is the Star Year (“SY”), which is when the Star Ratings are officially assigned. In the fourth year — the quality bonus payment (“QBP”) year.4
Understanding the New Star Ratings Measures
CMS is introducing five new measures across several domains, reflecting its desire to address critical areas of patient safety, mental and physical health. By focusing on these key areas, CMS aims to drive improvements in better care outcomes, reduce unnecessary healthcare utilization, promote a higher standard of care for all Medicare beneficiaries and improve health equity.5 These measures are designed to comprehensively encourage MA plans to be innovative in their approach to meet beneficiaries' diverse needs.
Concurrent Use of Opioids and Benzodiazepines (“COB”) — [NEW]
Measurement Period: January 1st to December 31st | Measure Weight: 1x
This measure evaluates the simultaneous prescription of opioids and benzodiazepines, which significantly increases the risk of respiratory depression and fatal overdoses. Compliance is determined by avoiding 30 or more cumulative days of overlapping prescription fills. The measurement period begins with the first opioid prescription claim, and exceptions are made for patients receiving palliative care or hospice during the measurement period.6
Polypharmacy: Use of Multiple Anticholinergic Medications in Older Adults (“POLY-ACH”) — [NEW]
Measurement Period: January 1st to December 31st | Measure Weight: 1x
This measure focuses on the risks associated with older adults taking multiple anticholinergic medications concurrently, which can lead to cognitive decline. Noncompliance is triggered by 30 cumulative days of overlapping prescription fills, and it specifically targets patients aged 65 and over.7
Kidney Health for Patients with Diabetes — [NEW]
Measurement Period: January 1st to December 31st | Measure Weight: 1x
This measure is intended to improve outcomes for diabetic patients by monitoring kidney health. It focuses on ensuring that MA plans provide necessary screenings and interventions to prevent kidney disease progression, a common complication in diabetic patients. It measures the percentage of members aged 18-85 with diabetes (type 1 and type 2) who received a kidney health evaluation during the measurement year. The evaluation is defined by an estimated glomerular filtration rate (“eGFR”) and a urine albumin-creatinine ratio (“uACR”). This measure uses NCQA Healthcare Effectiveness Data and Information Set (“HEDIS”) data as its primary data source.8
Improving/Maintaining Physical Health — [Weight Increase]
Measurement Period: July to November | Measure Weight: 3x
This measure assesses patients' physical health by monitoring changes in physical functioning over time. It aims to ensure that MA plans effectively support patients in maintaining or improving their physical health, preventing deterioration and promoting overall well-being. It is evaluated through the annual Health Outcomes Survey (“HOS”), which collects self-reported beneficiary health status and quality of life data. The HOS includes questions about physical functioning, pain and general health, providing a comprehensive picture of the patient's physical health over time.9
Improving/Maintaining Mental Health — [Weight Increase]
Measurement Period: July to November | Measure Weight: 3x
This measure focuses on the mental health status of patients. It evaluates how well MA plans support mental health maintenance or improvement, addressing issues such as depression, anxiety and other mental health conditions to enhance patients' quality of life. It is evaluated through the HOS survey, which collects self-reported beneficiary data regarding emotional well-being, such as the frequency of feelings of depression, anxiety and other mental health conditions. By addressing these mental health issues, the measure aims to enhance patients' quality of life and ensure that MA plans provide adequate support for mental health maintenance and improvement.10
Other Star Ratings Changes & Considerations
As part of CMS’s ongoing efforts to enhance healthcare quality and patient experience, the 2026 Star Ratings changes include several updates that reflect the agency’s evolving priorities. These adjustments are designed to address emerging healthcare challenges, incentivize high-quality care and ensure that MA plans are aligned with CMS’s strategic goals, but they have significant implications for payers, as they dictate the areas where plans must focus their resources and efforts to maintain or improve their Star Ratings.11 Understanding these changes is crucial for payers to effectively adapt their strategies and ensure compliance.
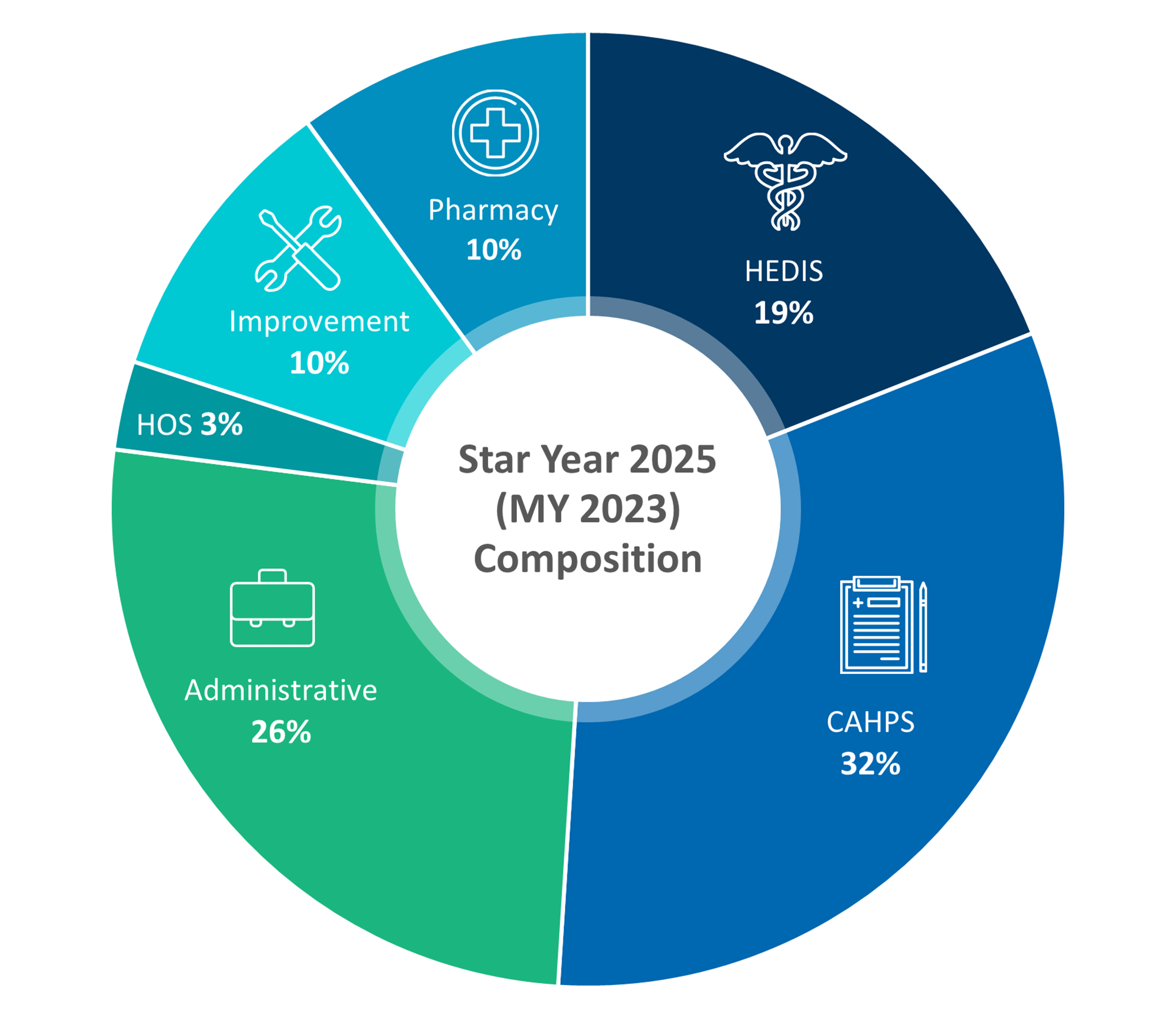
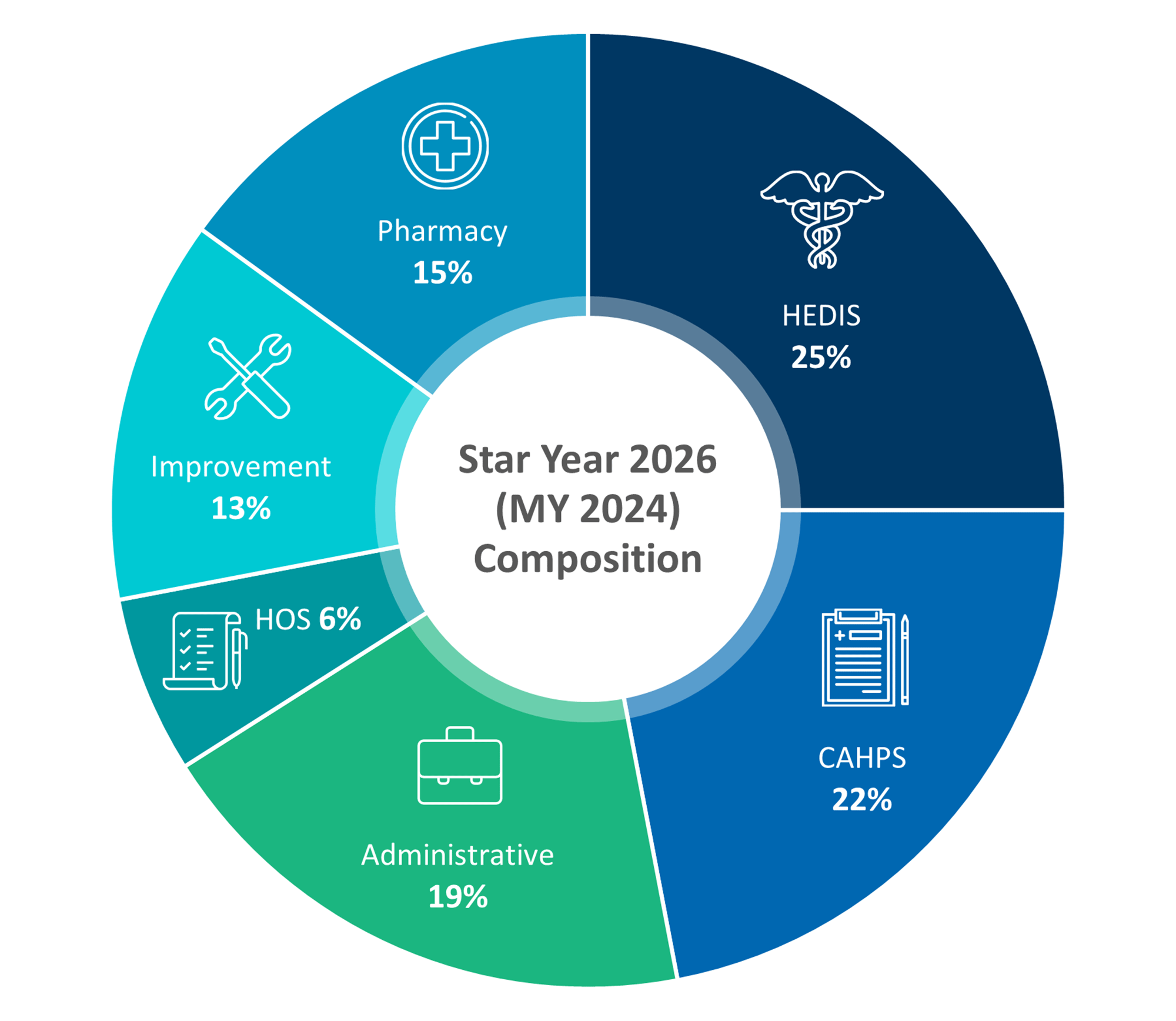
|
Source12 |
Source13 |
Change in Weight for CAHPS & Admin Measures
CMS will reduce the weight of CAHPS and administrative measures from 4x to 2x.14 This change shifts the focus slightly away from patient experience and administrative efficiency, prompting payers to balance their efforts across a broader range of quality measures. For payers, this adjustment means they must continue to prioritize patient interactions and administrative processes, but also increase emphasis on other critical areas, such as clinical outcomes and health equity.
Health Equity Index
Both MY 2024 and MY 2025 performance data will be used in calculating the Health Equity Index (“HEI”) reward applied to SY 2027 (published in October 2026). The HEI will replace the current reward factor — which rewards plans that have consistent high performance — with emphasis on the importance of equitable healthcare delivery. This index aims to reduce healthcare access and outcomes disparities among different demographic groups (e.g., Dually Enrolled (“DE”), Low-Income Subsidy (“LIS”) and/or Disability).15
Conversion of Colorectal Cancer Screening to Electronic Clinical Data Systems
The measure now uses electronic clinical data systems (“ECDS”) and eliminates hybrid reporting. ECDS streamlines the data collection process, reduces administrative burden and improves accuracy by integrating electronic health records, claims data and other digital sources.16
Modification of Breast Cancer Screening
This measure has been adjusted to be gender neutral. This change is significant, as it ensures inclusivity for all genders, acknowledging that breast cancer, though predominantly affecting women, can also impact men and transgender individuals. This adjustment aims to improve early detection and treatment outcomes across a broader population.17
Application of Continuous Enrollment Requirement
This criterion is now included for the Part D Medication Adherence and Statin Use in Persons with Diabetes (“SUPD”) measures to ensure consistency and accuracy in reporting. Continuous enrollment ensures that only patients with consistent coverage are counted in the denominator, leading to more reliable and actionable data.18
Continuous Enrollment and Hospice Exclusions
Continuous enrollment is now used for denominator inclusion, and hospice exclusions vary across measures. To avoid compliance issues, payers must keep reporting and analytics systems updated with these changes.19
Data Integrity Penalties
CMS has emphasized the importance of data integrity. Expanding Prescription Drug Event (“PDE”) reconciliation rigor to include these drug classes will help prevent CMS from reducing measure ratings to one Star due to data integrity violations.20
Strategic Insights for Success
Excelling at engaging members and closing the new and updated CMS Star Ratings measures provides several benefits to both payer and provider organizations, including enhanced patient care and outcomes, reduced longer-term healthcare expenditures and better alignment of Star ratings with measures that impact both cost and quality of care.21 FTI Consulting recommends that clients take the following actions to focus on the essential elements of clinical engagement, data integrity, patient-centered care and continuous improvement. Improving these areas can help MA plans deliver superior outcomes that align with CMS’s vision of high-quality, equitable healthcare.
Clinical and Provider Engagement
Leverage clinical or provider engagement resources when addressing the two new pharmacy measures (COB and POLY-ACH), rather than engaging a pharmacy team. Where clinically appropriate, deprescribing requires complex patient monitoring to avoid compromising patient health. Clients should develop protocols for identifying patients at high risk for concurrent opioid and benzodiazepine use and train providers on safe prescribing practices and deprescribing techniques.22
Data Validation
Use claims data instead of PDE data to help accelerate intervention times, which are critical for maintaining compliance.23 Claims data provides a more immediate and actionable source of information. FTI Consulting recommends implementing a system for regularly comparing claims data with PDE data to identify discrepancies and ensure data accuracy.
Data Strategy Differentiation
Employ retrospective data for reporting purposes and prospective data for real-time interventions. Utilize retrospective claims data to identify patients requiring interventions for the COB and POLY-ACH measures.24
Payers should leverage real-time patient data to identify patients with physical or mental health and implement preventive measures so that healthcare professionals can quickly intervene.
Dashboard Updates
Ensure that MY 2025 dashboards are updated to reflect the modifications to the new and existing measures. Removal of measures with substantive changes, such as the Medication Therapy Management (“MTM”) measure, and the introduction of new ones, such as COB, POLY-ACH and Kidney Health for Patients with Diabetes, require integrated planning and projections.25
Conclusion
Plans that actively innovate and continuously improve their operations tend to be the high achievers in terms of Star Ratings and other key performance metrics. CMS additions and modifications to MA and Part D Star Ratings measures will require plans to make operational enhancements to achieve gains in quality and equity of healthcare.
With 2025 on the horizon, health plans should execute against their operational roadmaps and be ready to execute CMS’s requirements starting January 1, 2025.
Footnotes:
1: CMS, “2025 Medicare Advantage and Part D Advance Notice Fact Sheet,” CMS (2024).
2: Ibid
3: eCFR Chapter 42, Part 422 Subpart D (7/17/2024).
4: Ibid
5: CMS, “Advance Notice of Methodological Changes for 2025 for Part C & D Payment Policies,” CMS (01/31/2024).
6: CMS, “Medicare 2024 Part C & D Display Measure Technical Notes,” CMS (12/14/2023).
7: Ibid
8: Ibid
9: Ibid
10: Ibid
11: CMS, “2025 Medicare Advantage and Part D Rate Announcement Fact Sheet,” CMS (04/01/2024).
12: Ibid
13: CMS, “2026 Star Ratings Measures,” CMS (07/29/2024).
14: CMS, “2025 Medicare Advantage and Part D Rate Announcement Fact Sheet,” CMS (04/01/2024).
15: CMS, “Advance Notice of Methodological Changes for 2025 for Part C & D Payment Policies,” CMS (01/31/2024).
16: CMS, “2025 Medicare Advantage and Part D Rate Announcement Fact Sheet,” CMS (04/01/2024).
17: Ibid
18: Ibid
19: Ibid
20: Ibid
21: Optum, “2025 CMS Advance Notice Executive Summary,” Optum (2024).
22: Ibid
23: Ibid
24: Ibid
25: CMS, “2025 Medicare Advantage and Part D Rate Announcement Fact Sheet,” CMS (04/01/2024).
Related Insights
Related Information
Published
September 04, 2024