mRNA: An Advanced Technology for Precise Yet Agile Patient Care
-
November 19, 2024
-
Messenger RNA (“mRNA”) technology represents a transformative advancement in vaccine development, offering several advantages over traditional vaccine platforms. The rapid success of mRNA vaccines during the COVID-19 pandemic demonstrated their efficacy as well as their potential to revolutionize how vaccines are developed and manufactured for a wide range of diseases. When compared to traditional vaccines, mRNA technology offers significant benefits in terms of speed, flexibility, safety and scalability, making it a superior option for future vaccine development. FTI Consulting believes the mRNA platform continues to face various operational challenges that must be addressed to ensure long-term viability and scalability.
In recent years, mRNA technology has emerged as a revolutionary platform in the development of vaccines. While conventional vaccine platforms have played a critical role in global immunization efforts, mRNA technology has demonstrated its potential to transform vaccine development and delivery.In this article, we explore FTI Consulting’s view on how to best capitalize on these successes as an organization and as a healthcare community. More specifically, we discuss advancements in speed, flexibility, safety and potential while exploring challenges related to distribution, cost and public perception.
Speed of Development, Manufacturing and Reformulation
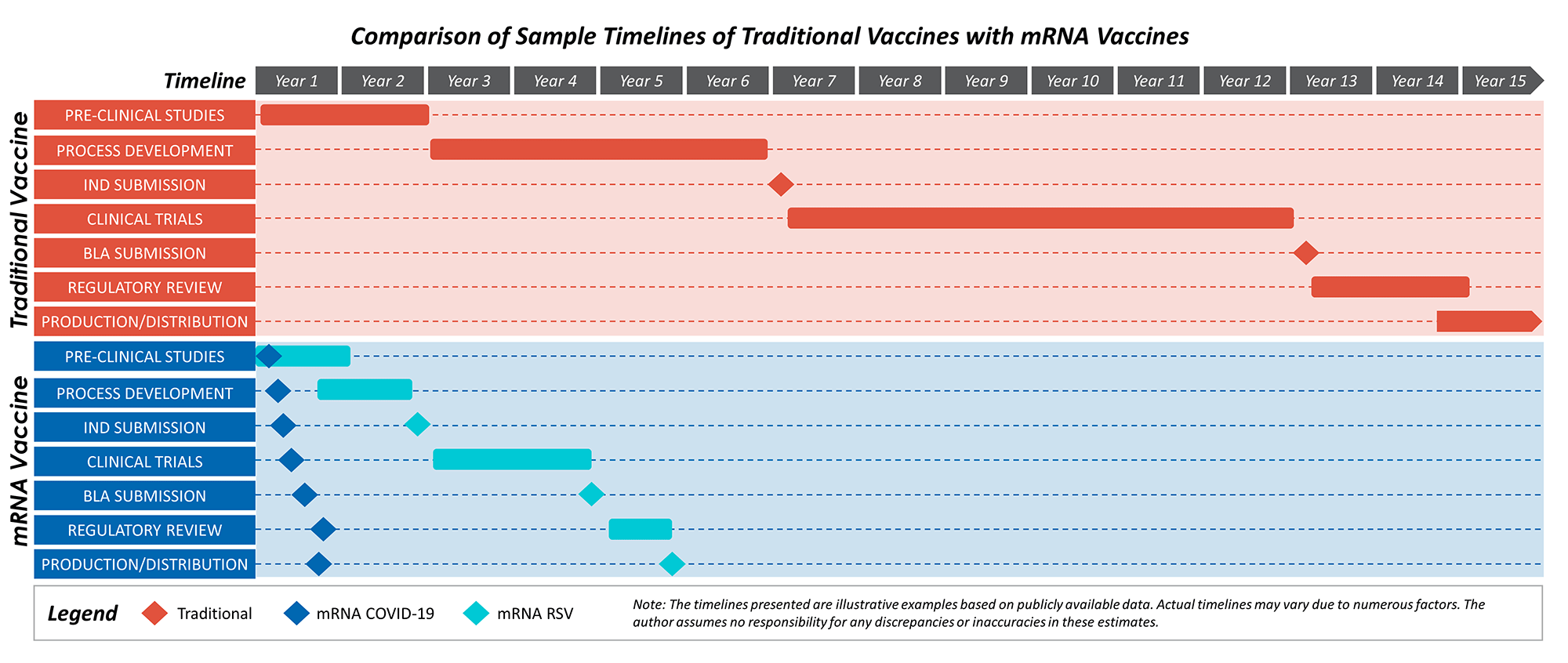
One of the most compelling advantages of mRNA technology is the speed at which vaccines can be developed and produced. Traditional vaccines, such as inactivated or live-attenuated vaccines, rely on growing pathogens in a lab, which is time-consuming and resource-intensive.1 The entire process takes 10–15 years, on average, whereas mRNA vaccines have fewer production steps and do not require cultivating the virus or growing pathogens.2 During the COVID-19 pandemic, mRNA vaccines from Pfizer-BioNTech and Moderna were developed and approved for emergency use within a record-setting nine months and 11 months, respectively.3
While the urgency of the COVID-19 pandemic contributed to an expedited developmental timeline by leveraging pre-existing studies, parallel efforts/trials, expedited regulatory reviews and at-risk production, the speed of development of mRNA vaccines was proven as a realistic and viable capability. The development introduced mRNA as a strong platform in the vaccine space due to its adaptability and manufacturing ease. The mRNA RSV vaccine produced by Moderna did not have the same urgent public health crisis or emergency authorizations as the vaccine for COVID-19, but it still took only five years from development to approval in May 2024, as detailed in Figure 1.4
Figure 1: Sample Timelines for Traditional Vaccines vs. mRNA Vaccines
Figure 1: Comparison of timelines for traditional vaccine development vs. mRNA vaccine development by mapping out the estimated time per developmental step for each type of vaccine, noting that while traditional vaccine development can take upwards of 15 years, mRNA COVID-19 took under a year and mRNA RSV took under six years.5, 6
A critical component of the development timeline is the lead time to produce. While a traditional vaccine lot ranges from several months to three years including quality control, with mRNA technology, once the sequence is known, large batches can be manufactured in a short time using standardized, cell-free processes.7 For example, it takes three to seven days for BioNTech to produce mRNA batches, with four to five weeks for quality control.8
Furthermore, mRNA vaccines can be adapted quickly to address new variants of seasonal viruses or with emerging infectious diseases. It took Moderna only 30 days to prepare a reformulated version of its vaccine for trials in 2021, compared to approximately five months of reformulation required for traditional biological vaccines.9 We believe that as mRNA development progresses, areas like supply chain, regulatory approval needs and clinical operations, but not R&D, will be the bottlenecks. To mitigate these risks, companies must adapt and streamline core processes to enable a faster and more scalable path to commercialization.
This accelerated nature of mRNA development requires different approaches to product lifecycle management, particularly as regulatory expectations around these vaccines evolve. Based on FTI Consulting’s experiences with mRNA manufacturers, as seasonal or new vaccine formulations are rapidly updated and company portfolios become more complex, there is also a rising need for proactive master data management to enable the expedited timelines of development and manufacturing.
Flexibility and Pandemic Preparedness
For seasonal vaccines, the FDA works closely with manufacturers and health organizations — as early as nine months beforehand — to identify the most likely strains that will circulate. However, with the traditional seasonal vaccine model, such as for influenza, the sourcing and manufacturing process often commences before FDA approval is received to ensure that vaccines can be available for the public in time for the flu season.10
In the case of global health emergencies, as seen in the COVID-19 pandemic, billions of doses were required in a compressed timeframe, and there were shifts in variants mid-pandemic. These shifts had major supply chain ramifications, yet these are challenges that all traditonal and mRNA vaccines could be subject to. For example, the recent decision by the FDA’s VRBPAC to shift the focus to the KP.2 strain for the 2024-2025 COVID-19 vaccine formula in June 2024 disrupted supply chains for manufacturers that had been preparing for the previously recommended JN.1 strain.11
To prepare for similar challenges, FTI Consulting has worked with manufacturers to implement agile supply chain management capabilities that are not widespread in the pharmaceutical industry. This includes flexible manufacturing practices and proactive scenario planning to help ensure that both production schedules and capacity mirror evolving demand. Since mRNA is highly adaptable and quick to produce, this technology allows governments and manufacturers to take the guesswork out of both seasonal strains and future pandemics to ultimately reduce the cost and risk of manufacturing materials for vaccines that may never be approved. Public health organizations, such as the CDC, FDA and WHO, should work simultaneously to update their policies (e.g., purchasing protocols, efficacy) to meet the shifts in vaccine technology that now allow for just-in-time, accurate operations at the right scale.
Safety Profile
Traditional vaccines often require the use of live-attenuated or inactivated viruses, which carry inherent safety risks, particularly for immunocompromised individuals. In contrast, mRNA vaccines do not use any live viruses, and therefore pose no risk of introducing disease or infectious elements into the body. The mRNA itself is non-infectious and is rapidly degraded by the body after the encoded protein is produced. This safety profile makes mRNA vaccines suitable for a wide range of populations and therefore is a distinct advantage. The safety requirements in the mRNA manufacturing process are also less stringent when compared to live or inactivated vaccines. which require significant considerations for biosafety regulations. Therefore, mRNA production facilities can be smaller, faster and more cost-effective to launch.12, 13
Although mRNA vaccine production is faster and more cost-effective than alternative methods, there remains a continuing challenge in vaccine development and production: reducing timelines and resources needed for quality testing while maintaining rigorous quality assurance standards to ensure patient safety. We have seen success in this space across teams that empower their people to automate quality-control processes, implement real-time monitoring and adopt a risk-based approach to quality management. Adjusting their quality management systems to account for the substantially different approach of manufacturing mRNA vaccines will further allow manufacturers to benefit from the reduced timelines while maintaining patient safety.
Future Application Potential
mRNA technology allows for precise targeting of antigens such as the spike protein in the case of COVID-19, enabling a highly targeted and well-defined immune response. In particular, due to mRNA’s ability to encode virtually any protein, researchers are exploring its use in creating custom vaccines that can teach the body’s immune system to recognize and attack cancer cells.14 This opens up a world of possibilities for mRNA to revolutionize not just vaccine science, but precision medicine as a whole.
The mRNA platform is flexible enough to serve patients with billions of doses while also offering promise for developing personalized cancer vaccines and therapies for rare genetic diseases. A number of companies have recognized these benefits and are increasing their investments of time and resources in the mRNA space. For example, in the oncology space, Phase III trials are underway for an mRNA combination treatment with Keytruda for high-risk melanoma and non-small cell lung cancer through a collaboration between Merck and Moderna. The mRNA combination treatment reduced risk of recurrence or death by 49% in Phase IIb trials for melanoma when compared to Keytruda alone. In other oncology trials, BioNTech and Roche/Genentech are testing mRNA products in combination with chemotherapy.15 For treatment of cystic fibrosis, Vertex and Moderna are collaborating for the delivery of gene-editing therapies and have annouced the clearance of their related IND application for VX-522 in late 2022.16
Challenges Impacting mRNA Accessibility
While there are many exciting collaborations in this space, there are a few key challenges.
Distribution
mRNA technology typically has restrictive cold chain distribution requirements (e.g., –70°C needed for Pfizer/BioNTech COVID-19 vaccine stability), which pose logistical difficulties for transportation and storage, particularly in low-resource settings. The short shelf-life, complexity of packaging, and storage for various delivery methods also pose inherent challenges.17 While pre-filled syringes (“PFS”) are most widely used and relatively straightforward for distribution, alternate methods such as nasal sprays, microneedle patches and inhalers require specialized packaging and handling.
Cost
Overall mRNA cost per dose poses a risk to global access due to factors such as higher raw material cost, uncertainty of new technology and lack of dedicated manufacturing infrastructure. mRNA vaccine doses range from $15 to $30, while traditional vaccines can cost as low as $2-$10 per dose.18, 19
Public Perception
The public perception of mRNA vaccines has evolved significantly since their introduction, especially as a result of the COVID-19 pandemic. While they have been largely seen as a groundbreaking achievement in vaccine technology, opinions about mRNA vaccines vary widely depending on factors such as geography, personal beliefs, political views and exposure to information. A 2024 survey from the Global Listenings Project (“GLP”) found that comfort in innovations in mRNA medicines was generally low (35%).20
Evolving Solutions
Despite these considerable challenges, innovative approaches are emerging to enhance mRNA vaccine accessibility, reduce distribution costs and build public confidence in this transformative technology.
Distribution Requirements
Advances in formulation and storage technologies are being developed to overcome distribution hurdles, making mRNA vaccines more accessible globally. For example, Moderna’s COVID-19 formulation is now stable at refrigerator temperatures for 30 days while Pfizer’s is stable at refrigerator temperatures for up to 10 weeks.21, 22 In our experience, product portfolio and external partnership management should be used to negate the risks posed by these requirements from the early clinical phase and throughout the product lifecycle.
Although mRNA development and manufacturing is highly effective and scalable, companies need to ensure they are also maintaining the proper partnerships (e.g., CMOs, third-party couriers) to promote dynamic distribution needs as well. We believe that storage, warehousing and transportation capacity need to be equally scalable to realize the full benefits across a variety of packaging types and ongoing shifts in the regulatory (i.e., shipping validation) landscape. Solutions should focus on streamlining packaging and simplifying device design for more efficient storage and transport, while also balancing consumer design requirements for ease of administration.23
Cost
While much of the expense of vaccines is currently being subsidized by governments, this should decrease over time as economies of scale and manufacturing capabilities increase. As the platform becomes more proven and commercially successful, supply chain teams should work to optimize operations on a variety of manufacturing and logistics fronts. With increasing adoption and consumption of mRNA products, economies of scale and platform developments are likely to minimize these challenges.
Public Perception
Public confidence around mRNA technology remains low, but various efforts are underway to improve this perception. FTI Consulting’s Strategic Communications team has seen great success in increasing public awareness of new technologies by developing strategic communication campaigns, developing patient advocacy programs, and aligning with regulatory agencies for public safety campaigns to ultimately build deeper trust in the technology and stronger brand perception.
Overall: A Game-changer in Vaccine Technology
mRNA technology represents a paradigm shift in vaccine development, offering advantages in speed, safety, flexibility and scalability over traditional vaccines. Its ability to rapidly adapt to new viral threats, combined with its robust safety profile, makes it an ideal platform for combating future pandemics and emerging diseases. While challenges remain, continued advancements in mRNA technology are likely to position it as the future of vaccine science, as well as a standard platform with applications far beyond infectious diseases, including cancer and other therapeutic areas.
This breakthrough in biotechnology promises not only to improve public health outcomes but also to redefine what is possible in preventive medicine, making mRNA vaccines a superior and future-focused solution far beyond a response to a global emergency. Let’s stay ahead of it: FTI Consulting is committed to bringing operational, financial and strategic communications expertise to making mRNA technology more widely adopted and accessible, as well as an increasingly reliable method for developing vaccines.
Reach out to one of our FTI Consulting experts to learn more and to equip your company to stay ahead in the ever-evolving biotech space.
Footnotes:
1: Ghattas, Majed, et al. “Vaccine Technologies and Platforms for Infectious Diseases: Current Progress, Challenges, and Opportunities.” Vaccines, U.S. National Library of Medicine (16 Dec. 2021).
2: Han, Seunghoon. “Clinical Vaccine Development,” Clinical and Experimental Vaccine Research 4, no. 1 (January 2015): 46–53.
3: Thorn, Chelsea R., Divya Sharma, Rodney Combs, Sonal Bhujbal, Jennifer Romine, Xiaolu Zheng, Khurram Sunasara, and Advait Badkar. “The Journey of a Lifetime — Development of Pfizer’s COVID-19 Vaccine.” Current Opinion in Biotechnology 78 (December 2022): 102803.
4: Krammer, Florian. “SARS-CoV-2 Vaccines in Development.” Nature 586, no. 7830 (October 2020): 516–27.
5: “Use of Respiratory Syncytial Virus Vaccines in Adults Aged ≥60 Years: Updated Recommendations of the Advisory Committee on Immunization Practices — United States, 2024.” Centers for Disease Control and Prevention, Centers for Disease Control and Prevention (15 Aug. 2024).
6: “History of mRESVIA.” Drugs.com (October 4, 2023).
7: Plotkin, Stanley, James M. Robinson, Gerard Cunningham, Robyn Iqbal, and Shannon Larsen. “The Complexity and Cost of Vaccine Manufacturing – An Overview.” Vaccine 35, no. 33 (July 24, 2017): 4064–71.
8: “How to Make Enough Vaccine for the World in One Year,” Public Citizen (May 26, 2021).
9: Prabhala, Achal, and Alain Alsalhani. “Developing Countries Can Make the mRNA Vaccines They Need.” Nature Human Behaviour 6, no. 2 (February 2022): 167–167.
10: “FDA’s Critical Role in Ensuring Safe and Effective Flu Vaccines.” U.S. Food and Drug Administration.
11: “Updated COVID-19 Vaccines for Use in the United States Beginning Fall 2024.” U.S. Food and Drug Administration (September 25, 2024).
12: Ibid.
13: Baden, L. R., El Sahly, H. M., Essink, B., et al. (2021). Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. The New England Journal of Medicine, 384(5), 403-416.
14: “How mRNA Vaccines Might Help Treat Cancer - NCI.” cgvBlogPost (January 20, 2022).
15: “Could Cancer Vaccines Be the Next Big Breakthrough in Immunotherapy?” BioSpace (March 28, 2024).
16: “Vertex Announces Investigational New Drug (IND) Application for VX-522.” Vertex Pharmaceuticals (September 25, 2024).
17: “Pfizer-BioNTech COVID-19 Vaccine Storage and Handling Summary.” Centers for Disease Control and Prevention, (May 2, 2023).
18: Jennifer Kates, C. Cox, and J. Michaud, “How much could covid-19 vaccines cost the U.S. after commercialization?” KFF, (2023, March 10).
19: “Current CDC Vaccine Price List.” Centers for Disease Control and Prevention, Centers for Disease Control and Prevention (1 Oct. 2024).
20: Iqbal, Shehzad M., et al. “Opportunities and Challenges to Implementing mRNA-Based Vaccines and Medicines: Lessons from Covid-19.” Frontiers in Public Health (12 July 2024).
21: Uddin, Mohammad N, and Monzurul A Roni. “Challenges of Storage and Stability of mRNA-Based COVID-19 Vaccines.” Vaccines, U.S. National Library of Medicine (17 Sept. 2021).
22: “Pfizer-BioNTech COVID-19 Vaccine.” Pfizer-BioNTech COVID-19 Vaccine Information | CDC (4 Jan. 2021).
23: Muthumani, K., and D. J. Weiner. “Development and Delivery Systems of mRNA Vaccines.” Frontiers in Immunology 12 (2021): 774942.
Related Insights
Related Information
Published
November 19, 2024
 Key Contacts
Key Contacts
Senior Managing Director
Director
Senior Consultant
Most Popular Insights
- Beyond Cost Metrics: Recognizing the True Value of Nuclear Energy
- Finally, Pundits Are Talking About Rising Consumer Loan Delinquencies
- A New Era of Medicaid Reform
- Turning Vision and Strategy Into Action: The Role of Operating Model Design
- The Hidden Risk for Data Centers That No One is Talking About