Is your Company Equipped to Deliver Personalized Therapies?
Three Critical Steps to Establish an Effective Chain of Identity for Precision Medicine
-
August 30, 2022
DownloadsDownload Article
-
When commercializing a personalized therapy, investing in a chain of identity should be a top priority because its foundational elements ensure safe and successful patient outcomes. Chain of identity confirms that patients receive treatments specifically tailored to them, by leveraging advanced cell and tissue tracking. As a result, patients receive treatments that adhere to the strictest regulatory and quality standards.
The Life Sciences team at FTI Consulting recommends that chain of identity capabilities are established by executing three sequential steps:
- Map out and document the end-to-end processes that are being tracked and define the required capabilities
- Outline the stakeholders and their roles along the end-to-end value chain, from patient referral to long-term follow-up
- Identify the technology that best integrate the processes and needs of the end-users
These steps create focus and prioritization in order to meet critical timelines that relate to regulatory filing and approvals. In personalized medicine, the process is a leading indicator of the final product; therefore, the process should inform how stakeholders and technology interact, not the other way around.
Step 1. Map out and document the end-to-end processes that are being tracked and define the required capabilities
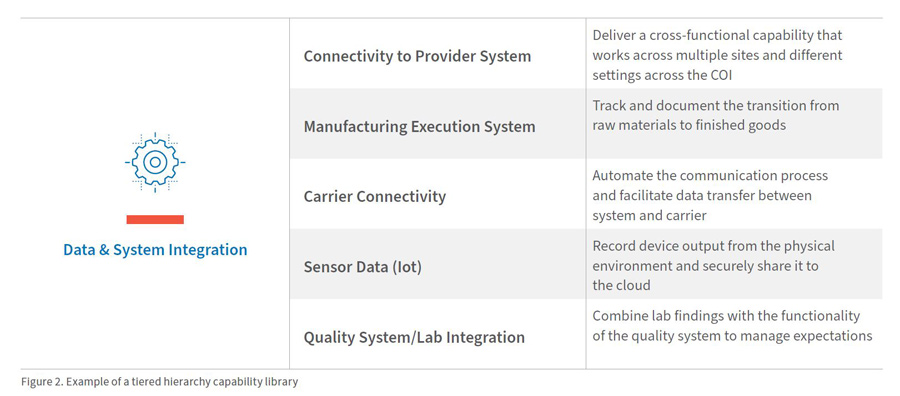
Companies should begin by creating a detailed, end-to-end process map that depicts each step of the process, beginning with patient enrollment and ending with long-term, post-treatment follow-up. These processes will help identify key stakeholders, critical third-party partnerships and care management organizations (CMOs), and information flows throughout the global network. Although the high-level steps might be similar across various treatment areas (see Figure 1), the detailed process steps are always unique because they are driven by the inherent complexity of the therapy in scope.
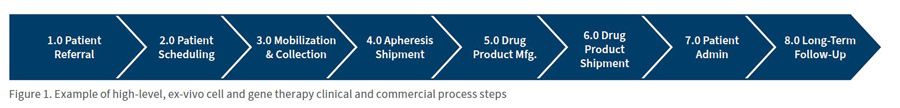
As pharmaceutical companies build their chain of identity, they should create a tiered capability hierarchy separated into primary, secondary and non-essential chain of identity capabilities. Figure 2 illustrates the connection between the modules and capabilities and how some capabilities are more critical than others in establishing an effective chain of identity.
Companies should clearly differentiate capabilities that drive core functions and those that are simply “nice to have.” It may seem trivial at first, but many implementations fail or are delayed because of convoluted expectations or lack of focused objectives. For instance, capabilities such as scheduling, labelling, third-party integration, and logistics management are often included in chain of identity solutions, these capabilities might complicate the core objective: tracking the individual’s cells.
Step 2. Outline the stakeholders and their roles along the end-to-end value chain, from patient referral to long-term follow-up
The most effective solutions will be designed with the end-users in mind. These solutions have easy-to-navigate user interfaces, advanced language capabilities, and they can be accessed from any location. The chain of identity environment becomes more complicated as the number of involved stakeholders increases, and the best solutions will reflect that by being scalable to the size of the organization. Implementing a solution without consulting the apheresis nurses, manufacturing personnel and physicians who will use it every day would be an oversight.
The end-users are arguably the most important part of the chain of identity, and they should be able to be trained quickly. It is important that users are guided to input timely and accurate information, but equally important is that they can easily access critical information. The solution should actively preempt complications that result from international (differentiated) business practices while maintaining consistency throughout the platform. It should have intuitive solution navigation and comprehensive feedback for user actions. The solution can then allow user-specific permissions based on role, seniority level, location and other criteria to ensure that the chain of identity is securely maintained, and potential human errors are reduced.
Step 3. Identify the technology that best integrates the processes and needs of the end-users
When it comes to the software-side of chain of identity, there are plenty of emerging and evolved providers to choose from. Companies in the process of selecting a solution partner should consider prior experience in the precision medicine space, the level of centricity that chain of identity has in the end-to-end process, and if the provider has similarly prioritized capabilities as selected in earlier steps.
Ultimately finding a solution partner who understands the symbiotic relationship between the users and the capabilities is necessary to developing a robust chain of identity. Each Solution has its own differentiated advantage and focus but it is important that the software can adapt to changing variables and relay those variables to the people using the system and to any third parties involved.
Chain of identity has quickly become an essential supply chain component for companies planning to be competitive in cell and gene therapy. Reinforced by the three critical steps: process and capability mapping, integrating end users, and identifying technological enablement, precision medicine companies will be able to deliver life-changing medicines — and drive bottom-line growth in their individualized therapies segments.
Related Insights
Related Information
Published
August 30, 2022
 Key Contacts
Key Contacts
Senior Managing Director
Downloads
Most Popular Insights
- Beyond Cost Metrics: Recognizing the True Value of Nuclear Energy
- Finally, Pundits Are Talking About Rising Consumer Loan Delinquencies
- A New Era of Medicaid Reform
- Turning Vision and Strategy Into Action: The Role of Operating Model Design
- The Hidden Risk for Data Centers That No One is Talking About