- Home
- / Insights
- / FTI Journal
- / Where in the World Is “Joe’s“ Blood?
Where in the World Is “Joe’s” Blood?

-
August 30, 2022
-
Cell and gene therapy is revolutionizing healthcare. But its efficacy relies on good old-fashioned supply chain management. Good luck with that.
Take a seat, Joe. We’re going to draw a vial of your blood and ship it to the lab for gene splicing. We expect to have it back here in the office in exactly seven days and we'll re-inject it then. That should cure your cancer.
That fantastic scenario is still way off in the future, but a variation of it is within sight. With recent developments of sophisticated gene-editing tools like CRISPR, scientists are getting closer to treating life-threatening diseases like cancer, diabetes, and sickle cell anemia through cell and gene therapies that correct flaws in our DNA.
It’s precision medicine at work — the innovative treatment tailored specifically to the genetic characteristics of the individual. We can expect these miracles to be delivered economically and quickly.
That is, if the providers get the right product into the right arms. For all the wonder of cell and gene therapy, the question about its efficacy hangs on something seemingly mundane, yet extremely critical: the end-to-end supply chain.
Handle with Care
As anyone who has confronted the empty store shelves so prevalent during the pandemic can attest, an efficient and reliable supply chain is vital for meeting customer demand.
It’s even more important, however, when you’re talking about people’s lives.
Precision medicine demands just that: precision. It demands expert knowledge and careful handling of vulnerable genetic material at every step in a circular process that starts and ends with Joe. That requires people — from the carriers overseeing the controlled containers, the technicians handling Joe’s cells, the return of what is now a drug product to the carriers, and those back at the medical center waiting to reinject Joe. And, of course, there’s Joe himself.
Precision and visibility are crucial for making sure Joe’s blood cells are handled by the strict dictates of his treatment protocol. Speed is of the essence; maintaining and preserving the “contents” throughout and delivering them expediently with the guarantee that no one else’s genetic material has “infected” the product is paramount.
Clearly, it’s not your grandparents’ supply chain. You can’t scale up by hiring more drivers, renting more warehouses, or throwing another box of widgets on the pallet. Problem with the product? There’s no label you can print out for returns. This supply chain, or circle, is literally a life-and-death operation and not every company in the precision medical field is ready.
As this bold new world dawns and more businesses rush to enter the market, the question then becomes: What do all parties along the supply chain need to know? And do they even know what they don’t know?
The Chain of Identity
The answers begin with understanding the concept of chain of identity (COI), the process on which all other elements in the precision medicine supply circle depends. Get it right and no one will probably notice. Get it wrong? You can kiss Joe goodbye.
Cell Power Tiny but powerful cells and genes are the engines that make precision medicine possible — the sparks that foster life-saving drug products. Joe can receive an autologous treatment for cancer that is derived from his own body for him and him alone. Or he can donate cells and genes that become the genesis for allogeneic drug products that will save thousands, millions even. Cells can come from the blood, bone marrow, a placenta, or elsewhere in the body.
COI requires mapping Joe’s treatment protocol at every step to ensure integrity, from blood draw to re-injection. You’ve got to identify the key people involved (e.g., stakeholders, critical third parties, CMOs) and monitor how information flows among them. And though you might be aware of the high-level steps involved with the process, you can’t lose sight of the lower-level steps unique to Joe’s treatment and those driven by the type of therapy in question.
Every treatment is one-of-a-kind. Cells can come from various areas of the body and be drawn in different ways, making the steps and people involved just as diverse. That differentiation means less consistency in the process and more room for error. No one wants this high-level operation to fail because an apheresis nurse (the expert in blood withdrawal) misses a small step or enters data about the recipient’s bone marrow incorrectly. (For more about sourcing in precision medicine, see sidebar “Cell Power.”)
Identify Your COI Capabilities
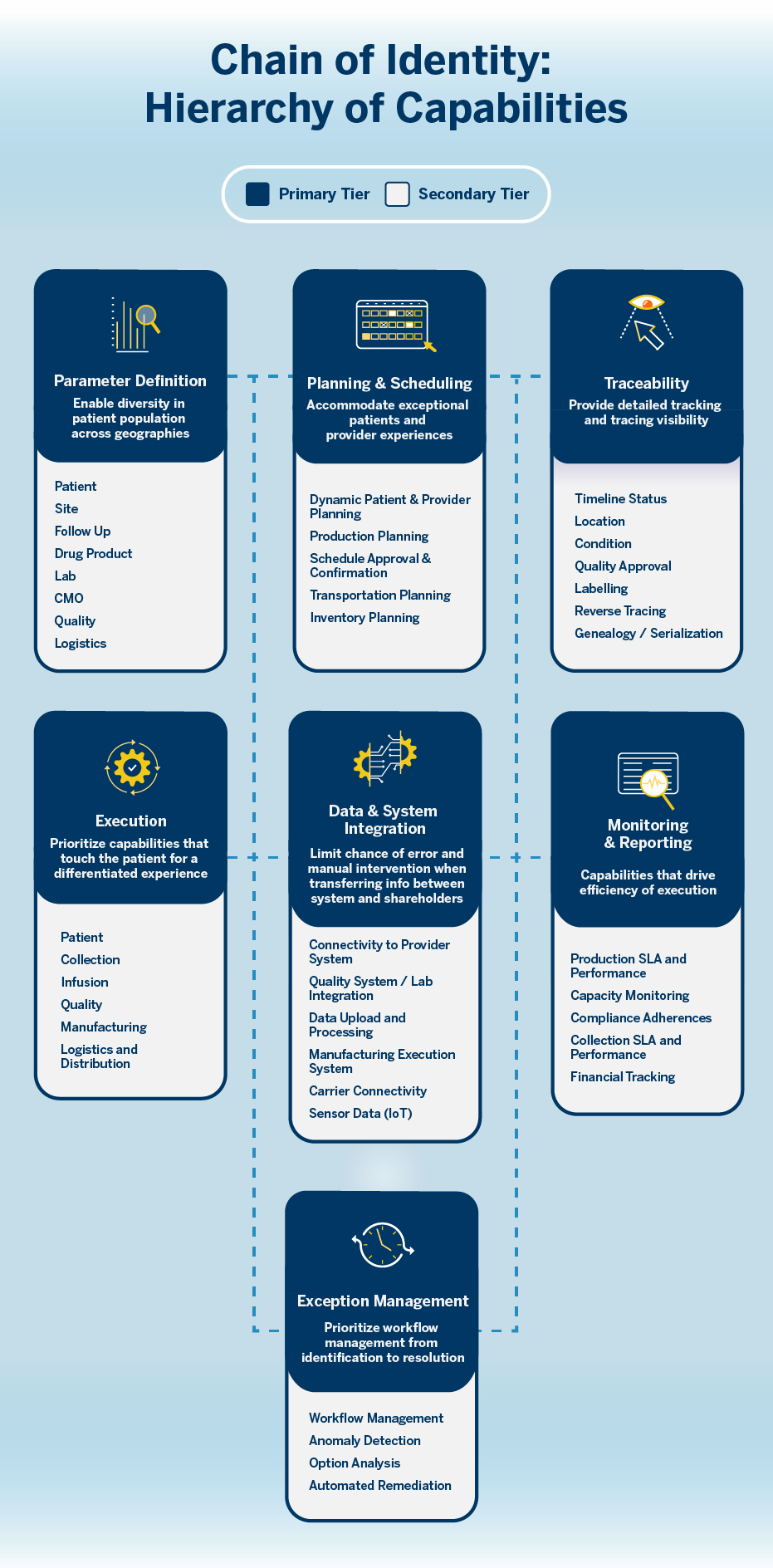
To establish a precisely functioning COI, it helps to look at a tiered hierarchy of capabilities in the supply chain process separated into primary, secondary, and non-essentials. From there, companies can evaluate where they stand in their ability to maintain the chain. They can rank capabilities that drive core functions and relegate others as “nice-to-have” for the moment. (See graphic below, “Chain of Identity: Hierarchy of Capabilities.”)
As organizations establish the COI as a critical component of operations, the key to success is to focus on the capabilities that truly drive patient outcomes. Secondary objectives are irrelevant.
After reviewing capabilities, the gaps in your COI should become apparent. Filling them is essential in a booming, rapidly evolving field where opportunities to expand are myriad. You may in fact already have advantages over the competition, but the future is coming on so fast that today’s cutting-edge business can quickly become last year’s model in a heartbeat.
“Realizing ideas requires investment, and to an increasing extent, partnerships and collaboration to ensure scale, reduce risk, and harness new technologies. The ability to effectively collaborate will become a growing source of competitive adantage.” — Denise Torres, board member, biopharma
That phenomenon makes it important to identify partners who not only understand this complex field but who are embedded in it. To make the most of an investment, and reduce the risk of failure, you want to avoid the growing pains that newcomers often experience.
Denice Torres, a board member for various biopharma companies, stresses the need for partnering in the gene & cell therapy industry to unlock its promises. “Realizing ideas requires investment, and to an increasing extent, partnerships and collaboration to ensure scale, reduce risk, and harness new technologies,” she says. “The ability to effectively collaborate will become a growing source of competitive advantage.”
Pick Your Partners
The key then, is to find partners experienced in precision medicine who understand COI and know how to prioritize capabilities. You want partners who can train people, put them in place and provide a solution-oriented approach that can quickly handle widely changing variables and eliminate — not just mitigate — risk.
The ability to communicate changes to everyone in the COI promptly, including third and, in some cases, fourth parties is a high priority. Products have to get out in the market promptly and within reasonable budgets. Your partner can help you get your COI buttoned-down, then assist with planning your end-to-end supply chain circle.
It’s no exaggeration to say that Joe’s life depends on nimbleness and adaptability. You don’t want to put him at further risk just because the number of cells collected from Joe was not what you anticipated needing at the start or the drug product you received back was insufficient.
It’s About the People
Your partner must ensure that your COI solutions and processes are designed with everyone on the chain in mind, including the most important party, Joe himself. Training is an absolute must. That requires keeping people up-to-date with protocols and changing products, how to incorporate these processes into what they are already doing so you don’t make their jobs harder, accounting for changing personnel, etc. Flexibility is key.
Ready for Delivery
COI has become part of the everyday vernacular in the precision medicine field, with companies working in cell and gene therapy truly needing to be able to grasp the complexities of implementing COI capabilities into their supply chains. By integrating the above processes, companies will be able to drive bottom-line growth. Most importantly, they’ll know where in the world Joe’s blood is every step of the way.
© Copyright 2021. The views expressed herein are those of the author(s) and not necessarily the views of FTI Consulting, Inc., its management, its subsidiaries, its affiliates, or its other professionals.
Watch Video
Cell and gene therapy is revolutionizing healthcare, but can your supply chain stand up to today’s risks? FTI Consulting can help your organization drive bottom-line growth, obtain required approvals and always know where Joe's blood is.
About The Journal
The FTI Journal publication offers deep and engaging insights to contextualize the issues that matter, and explores topics that will impact the risks your business faces and its reputation.
Published
August 30, 2022
Most Popular Insights
- Beyond Cost Metrics: Recognizing the True Value of Nuclear Energy
- Finally, Pundits Are Talking About Rising Consumer Loan Delinquencies
- A New Era of Medicaid Reform
- Turning Vision and Strategy Into Action: The Role of Operating Model Design
- The Hidden Risk for Data Centers That No One is Talking About


